Biotech Stock Roundup: PRAX, VKTX Up on Study Results & MRNA, REGN Give Updates
It was a busy week for the biotech sector, with lots of study data readouts and regulatory updates. Among these, Praxis Precision Medicines, Inc. PRAX and Viking Therapeutics, Inc. VKTX gain on study results.
Recap of the Week’s Most Important Stories:
Setback for Regeneron: Regeneron Pharmaceuticals, Inc. REGN suffered a setback as the FDA issued Complete Response Letters (CRLs) for its biologics license application (BLA) for odronextamab. The BLA is seeking the approval of the candidate in relapsed/refractory (R/R) follicular lymphoma (FL) and in R/R diffuse large B-cell lymphoma (DLBCL), each after two or more lines of systemic therapy.
Regeneron stated that the sole issue with approvability is related to the enrollment status of the confirmatory trials. The CRLs — one for R/R FL and one for R/R DLBCL — did not identify any approvability issues with the clinical efficacy or safety, trial design, labeling or manufacturing of the candidate. REGN has been actively enrolling patients in multiple phase III trials for odronextamab as part of the OLYMPIA program. The program is expected to change the treatment paradigm of several B-cell non-Hodgkin lymphoma subtypes, including in earlier lines of therapy.
The FDA agreed to the OLYMPIA program and required that the trials include both dose-finding and confirmatory portions. While enrollment has already begun in the dose-finding portion of the studies, the CRLs indicate that the confirmatory portions of these trials should be underway currently. The regulatory body also needs to agree to timelines prior to resubmission. Regeneron targets sharing updates on enrollment status and regulatory timelines later in 2024.
Praxis Up on Study Results: Praxis Precision Medicines, Inc. shares surged after the clinical-stage biopharmaceutical company reported positive results from its mid-stage study evaluating PRAX-628 in epilepsy patients with photo paroxysmal response (PPR).
PRAX-628 is a next-generation, functionally selective small molecule targeting the hyperexcitable state of sodium channels in the brain. It is currently being developed as a once-daily oral treatment for adult focal onset epilepsy. PPR studies measure electroencephalogram signatures after intermittent photic stimulation and are used as an indicator of anti-seizure efficacy. Two doses were evaluated in the phase IIa proof of concept study.
A total of six patients were baselined in the 15 mg cohort, of whom five were evaluable. One patient in the 15 mg cohort did not present adequate PPR at the baseline to be evaluated. Four patients were baselined in the 45 mg cohort, of whom three were evaluable. One patient in the 45 mg cohort was not evaluable due to lack of eligibility. Three patients from the 15mg cohort participated in the 45mg cohort after a washout period of greater than 100 days. Three patients were on background anti-seizure medications.
100% of patients achieved a complete response in the 45 mg cohort. 80% of patients achieved a complete response, and 20% achieved a partial response in the 15 mg cohort. Per management, the strength and consistency of response across both study arms, combined with a continued positive tolerability and safety profile, reinforces the potential of PRAX-628 to be the first precision sodium channel modulator for focal epilepsy patients. Consequently, Praxis plans to initiate an efficacy study in focal onset epilepsy on PRAX-628 in the second half of 2024.
Updates From Moderna: Moderna MRNA announced positive results from the phase III NextCOVE study evaluating mRNA-1283, its next-generation refrigerator-stable COVID-19 vaccine, in individuals aged 12 years and older. The study achieved its primary endpoint, showing that individuals who received mRNA-1283 generated a higher immune response against both the Omicron BA.4/BA.5 and original strains of SARS-CoV-2 compared to mRNA-1273.222, Moderna’s previously approved bivalent Omicron BA.4/BA.5-targeting COVID-19 vaccine. Per management, the benefit was primarily seen in older adults aged over 65 years, the population that remains at the highest risk of infection from the virus. Per Moderna, mRNA-1083 offers the potential for longer shelf life and storage advantages.
Moderna entered into a development and commercialization funding agreement with Blackstone Life Sciences to advance its flu program. As part of the agreement, Blackstone will fund up to $750 million with a return based on cumulative commercial milestones and low-single-digit royalties.
Moderna also announced updates demonstrating the advancement and acceleration of its mRNA pipeline. The company is advancing five vaccine candidates against viruses that cause latent infections. The phase III combination study of the company's investigational combination vaccine against flu and COVID-19 (mRNA-1083) for adults aged 50 years and older is fully enrolled, and data are expected in 2024. The company expects two more phase III readouts in 2024, including a combination vaccine against flu and COVID-19 and a vaccine against cytomegalovirus (CMV). Moderna announced positive clinical trial data from three new vaccines against viruses (Epstein-Barr virus, Varicella-Zoster virus, norovirus) and advanced programs toward phase III development.
Viking Up on Obesity Data: Shares of the clinical-stage biopharmaceutical company Viking Therapeutics, Inc. surged after the company announced positive results from an early-stage study on oral tablet formulation of VK2735 for obesity. The candidate is a dual agonist of the glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors in development for the potential treatment of metabolic disorders such as obesity.
The phase I study evaluated VK2735 at multiple dose levels and demonstrated dose-dependent reductions in mean body weight from the baseline after 28 days of daily dosing. Patients who received the drug at the highest dose level (i.e., 40mg) lost up to 5.3% of their body weight compared to 2.1% in the placebo group.
When adjusted for placebo rates, the drug reported a reduction in mean body weight of up to 3.3%. At the 20mg dose, patients lost weight by 1.1%. In addition, 57% of patients who received the 40mg dose of the drug lost at least 5% of their body weight. The drug was well-tolerated across all doses, with no serious adverse events.
Based on a preliminary evaluation of the above data, Viking believes that treatment duration beyond 28 days may provide further reductions in body weight. VKTX plans to initiate a phase II study of the candidate for obesity in the second half of 2024.
VKTX currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
The Nasdaq Biotechnology Index has dropped 1.16% in the past five trading sessions. Among the biotech giants, Moderna’s shares have risen 7.29% during the same time frame. Over the past six months, shares of GSK have surged 18.36%. (See the last biotech stock roundup here: Biotech Stock Roundup: GERN, CRNX Gain on Updates, BMY’s CAR T Cell Therapy News & More)
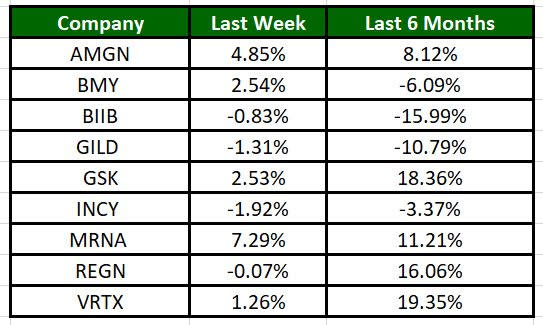
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Praxis Precision Medicines, Inc. (PRAX) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 