Disc Medicine (IRON) Down on Mixed Results From AURORA Study
Shares of clinical-stage biopharmaceutical company Disc Medicine, Inc. IRON plummeted 45% after the company announced mixed results from a phase II study of bitopertin in patients with erythropoietic protoporphyria (EPP).
The AURORA study is a randomized, double-blind, placebo-controlled phase II study that enrolled 75 adult subjects with EPP. The participants were randomized 1:1:1 to receive 20 mg of bitopertin, 60 mg of bitopertin, or placebo once daily for 17 weeks.
Bitopertin is the lead product candidate in the company’s heme biosynthesis modulation portfolio.
Treatment with bitopertin resulted in statistically significant reductions in toxic metabolite protoporphyrin IX (PPIX), the primary endpoint, and significant improvements in the rate of phototoxic reactions with pain and the Patient Global Impression of Change (PGIC).
The 60 mg dose reached statistical significance compared to placebo. The 20mg cohort achieved 21.6% reductions in whole blood PPIX levels, and the 60mg dose achieved a 40.7% reduction in whole blood PPIX levels, while the placebo group had mean increases of 8.0%.
However, the study did not achieve its key secondary endpoint of cumulative time in sunlight on days without pain. Results showed that biopterin-treated patients recorded a mean of 175.1 hours at 20 mg and 153.1 hours at 60 mg, compared with 133.9 hours for the placebo of cumulative total time in sunlight between 10 am and 6 pm on days without pain, as observed over the four-month treatment period.
While the magnitude of the improvement in the biopterin-treated patients was comparable to that observed in the BEACON study, the benefit in the placebo arm in the AURORA trial was greater than expected.
Bitopertin was generally well tolerated in both dose groups with no serious adverse events and stable hemoglobin levels.
Consequently, Disc Medicine will conduct an analysis of its final data set and work with investigators, regulators and patient advocacy groups to define the optimal registrational endpoints moving forward.
IRON’s shares have surged 56.1% in the past year against the industry’s decline of 7.1%.
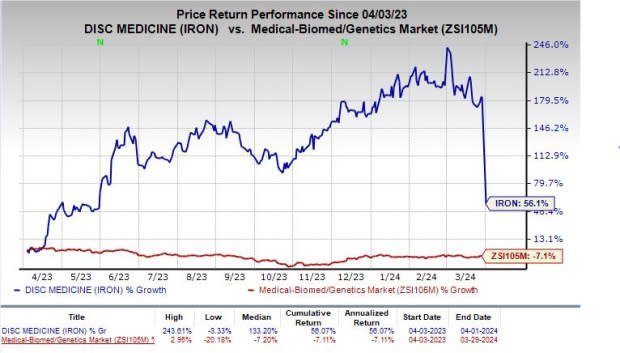
Image Source: Zacks Investment Research
Disc Medicine’s current pipeline includes bitopertin for the treatment of EPP and X-linked protoporphyria (XLP) and Diamond-Blackfan anemia, DISC-0974 for the treatment of anemia of myelofibrosis and anemia of chronic kidney disease, and DISC-3405 (formerly MWTX-003) for the treatment of polycythemia vera and other hematologic disorders.
While the results of the AURORA study were disappointing, the successful development of any of these candidates will be a big boost for the company.
Bitopertin was previously evaluated by Roche in a comprehensive clinical program in more than 4,000 individuals in other indications, which demonstrated the activity of bitopertin as a glycine transporter 1 and its effect on heme biosynthesis.
EPP and XLP are rare, debilitating and potentially life-threatening diseases caused by mutations that affect heme biosynthesis, resulting in the accumulation of a toxic, photoactive intermediate called PPIX.
At present, there is no cure for EPP. However, there is only one FDA-approved therapy — a surgically implanted synthetic hormone designed to stimulate melanin production called Scenesse (afamelanotide).
Zacks Rank & Stocks to Consider
IRON currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the biotech sector are ADMA Biologics, Inc. ADMA, Galapagos GLPG and ANI Pharmaceuticals, Inc. ANIP, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share (EPS) have improved from 22 to 30 cents. In the past year, shares of ADMA have surged 97%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met once, delivering an average surprise of 85.00%.
In the past 60 days, estimates for GLPG’s loss have narrowed from $1.68 per share to 40 cents.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 EPS have improved from $4.06 to $4.43. In the past year, shares of ANIP have surged 81.%.
ANI Pharmaceuticals’ earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 74.8%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Galapagos NV (GLPG) : Free Stock Analysis Report
Disc Medicine, Inc. (IRON) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 