Viridian (VRDN) Up 10% on Clinical Updates for Eye Disease Drug
Viridian Therapeutics VRDN has announced detailed plans for a late-stage program on VRDN-003, its investigational subcutaneously (SC)-administered antibody, in patients with moderate-to-severe thyroid eye disease (TED).
This August, management intends to start two late-stage studies, namely REVEAL-1 and REVEAL-2, evaluating two active dosing regimens of VRDN-003 in active and chronic TED, respectively. In both studies, patients will initially receive a high initial dose of 600mg (split into two 300mg injections). Following this dosage, patients will continue with injections of 300mg each. The total number of injections will depend on the treatment regimen – six injections in the four-week regimen and three injections in the eight-week regimen.
The primary endpoint of each study will be the proptosis responder rate (at least 2mm improvement in proptosis from baseline) after 24 weeks of treatment. Post this period, the study patients will be followed for another 28 weeks. Viridian intends to enroll around 84 and 126 patients in the REVEAL-1 and REVEAL-2 studies, respectively.
Viridian expects to report top-line data from both clinical studies in the first half of 2026. If data from the studies are positive, management plans to submit a regulatory filing with the FDA before 2026-end. If approved, the company plans to launch VRDN-003 with a commercially available autoinjector pen.
Following the announcement of the details, shares of Viridian rose 10.2% on Tuesday. Investors were impressed by the study particulars, with some Wall Street analysts praising the company’s execution plans.
If both studies are successful, the Viridian drug could be a strong contender to Amgen’s AMGN Tepezza, which is the first and only approved drug for TED. Unlike VRDN-003, which requires SC administration, Tepezza is administered intravenously (IV). Compared to IV drugs, SC drugs can be administered more comfortably and take much less time to be administered. Also, the VRDN-003 will require fewer dose administrations compared to the Amgen drug, which involves the administration of eight IV doses.
Year to date, Viridian’s shares have plunged 38.2% compared with the industry’s 5.6% fall.
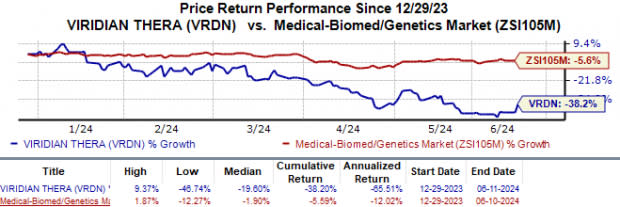
Image Source: Zacks Investment Research
Apart from VRDN-003, Viridian is evaluating its lead pipeline drug VRDN-001, an IV-administered antibody, in active and chronic TED in two separate late-stage studies, namely THRIVE and THRIVE-2, respectively. While top-line data from the THRIVE study is expected in September, data from the THRIVE-2 study is anticipated before this year’s end. If the outcomes from both studies are positive, Viridian expects to submit a regulatory filing for the drug with the FDA before 2025-end.
If VRDN-001 is approved, it is also likely to hold an edge over Tepezza. Though both drugs require IV administration, this Viridian drug entails less frequent dosing compared to the Amgen drug.
Per management, both VRDN-001 and VRDN-003 inhibit IGF-1R, which has been shown effective in treating TED. However, VRDN-003 has demonstrated a longer half-life of 40-50 days, which is four to five times compared to VRDN-001.
Amgen is also evaluating a SC formulation of Tepezza in a late-stage study in TED indication. Per a government website, the primary completion of this study is expected in September 2025.
Tepezza is part of Amgen’s rare disease franchise, which was added to its portfolio last year in October following the acquisition of Horizon Therapeutics for nearly $28 billion. The deal also added other rare disease drugs like Krystexxa and Uplizna to Amgen’s portfolio of marketed drugs.
Viridian Therapeutics, Inc. Price
Viridian Therapeutics, Inc. price | Viridian Therapeutics, Inc. Quote
Zacks Rank & Key Picks
Viridian currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector are Arcutis Biotherapeutics ARQT and Heron Therapeutics HRTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Arcutis Biotherapeutics’ 2024 loss per share have narrowed from $2.49 to $1.60. During the same period, the loss estimates per share for 2025 have narrowed from $1.77 to $1.14. Year to date, shares of Arcutis have surged 140.9%.
Earnings of Arcutis Biotherapeutics beat estimates in three of the last four quarters while missing the mark on one occasion. Arcutis delivered a four-quarter average earnings surprise of 14.93%.
In the past 60 days, estimates for Heron Therapeutics’ 2024 loss per share have narrowed from 22 cents to 10 cents. During the same period, estimates for 2025 have improved from a loss of 9 cents to earnings of 1 cent. Year to date, HRTX’s shares have appreciated 114.1%.
Earnings of Heron Therapeutics beat estimates in three of the last four quarters while missing the mark on one occasion. HRTX delivered a four-quarter average earnings surprise of 30.33%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Heron Therapeutics, Inc. (HRTX) : Free Stock Analysis Report
Arcutis Biotherapeutics, Inc. (ARQT) : Free Stock Analysis Report
Viridian Therapeutics, Inc. (VRDN) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 