Sarepta's (SRPT) DMD Drug sBLA Gets FDA's Priority Tag, Stock Up
Sarepta SRPT announced that the FDA has accepted its efficacy supplement to the biologics license application (BLA), seeking to expand the treatment label for its Duchenne muscular dystrophy (DMD) drug, Elevidys. The FDA has assigned the Priority Review status to the company’s Elevidys filing. A filing designated as a Priority Review reduces the review period by four months. A final decision from the regulatory body is expected on Jun 21, 2024.
The therapy was initially approved by the FDA in June 2023 under the accelerated pathway to treat ambulatory pediatric patients aged between four and five years with DMD. Following the accelerated nod, it became the first approved gene therapy for DMD.
The purpose of the efficacy supplement is two-fold. It seeks to expand the labeled indication for Elevidys to treat all DMD patients, irrespective of age and ambulation status. Subject to approval, it will also convert the Elevidys accelerated approval to a traditional approval.
Sarepta also reported that the regulatory body has confirmed that it will not hold any advisory committee meeting to discuss the efficacy supplement to the Elevidys BLA.
The company’s stock rose 7.8% on Feb 16 in response to the encouraging news. In the past year, shares of SRPT have gained 8.1% against the industry’s 10.6% decline.
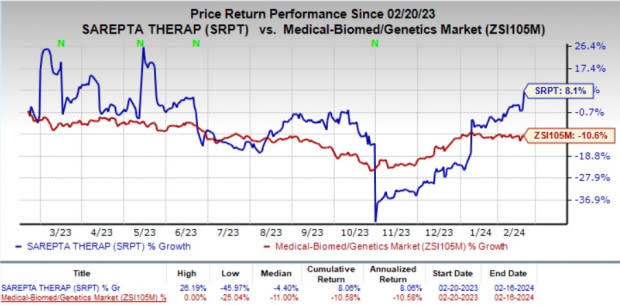
Image Source: Zacks Investment Research
The BLA supplement is mainly supported by data from the phase III EMBARK study, announced in October 2023, which also serves as the confirmatory study for Elevidys’ full approval in DMD indication. Though the study failed to achieve its primary endpoint, it gained statistical significance on all pre-specified key secondary endpoints, indicating that treatment with Elevidys modifies the course of DMD indication.
SRPT had discussed the above results with the FDA. Notably, the FDA had previously indicated that the regulatory body is open to reviewing the data for label expansion based on the totality of evidence from the EMBARK study.
Sarepta recently reported encouraging preliminary/unaudited results for the fourth quarter and full-year 2023. Per the company, Elevidys sales are expected to be around $131.3 million for the fourth quarter, bringing the total sales figure to around $200.4 million for the full year. This is an encouraging figure for a treatment that was commercially launched in third-quarter 2023.
SRPT developed Elevidys in collaboration with Roche RHHBY. Sarepta and Roche entered into a licensing agreement in 2019 to develop and commercialize Elevidys jointly. Per the agreement, Roche has exclusive rights to launch and commercialize the gene therapy in ex-U.S. markets. As part of the agreement, SRPT will also record collaboration revenues on the ex-U.S. sales made by RHHBY.
To satisfy regulatory requirements for Elevidys’ approval outside the United States, Sarepta is also evaluating the safety and efficacy of Elevidys in the ongoing phase III ENVISION study in non-ambulatory and ambulatory DMD patients.
Sarepta Therapeutics, Inc. Price and Consensus
Sarepta Therapeutics, Inc. price-consensus-chart | Sarepta Therapeutics, Inc. Quote
Zacks Rank and Other Stocks to Consider
Sarepta currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks from the drug/biotech industry worth considering are ADMA Biologics ADMA and Immunocore IMCR, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has remained constant at 2 cents. The consensus estimate for ADMA Biologics’ 2024 earnings per share is pegged at 22 cents. Over the past year, shares of ADMA have jumped 45.5%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 63.57%.
In the past 30 days, the Zacks Consensus Estimate for Immunocore’s 2023 loss per share has remained constant at 95 cents. During the same period, the consensus estimate for Immunocore’s 2024 loss per share has remained constant at $1.42. Over the past year, shares of IMCR have risen 11.7%.
IMCR beat estimates in two of the trailing four quarters and missed on the other two occasions. In the last reported quarter, IMCR reported an earnings surprise of 112.5%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Immunocore Holdings PLC Sponsored ADR (IMCR) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 