Ultragenyx (RARE) DTX401 Meets Goals in Metabolic Disorder Study
Ultragenyx Pharmaceutical RARE announced positive top-line results from the late-stage study of its investigational AAV8 gene therapy, DTX401, to treat glycogen storage disease type Ia (GSDIa) patients aged eight years and older. GSDIa is an inherited metabolic disorder that affects approximately 6,000 patients worldwide.
Per the data readout, the phase III GlucoGene study (NCT05139316) achieved its primary endpoint demonstrating a statistically significant and clinically meaningful reduction in daily cornstarch intake upon treatment with DTX401 compared with placebo at week 48.
In patients treated with the gene therapy candidate, a mean percent reduction of 41.3% was observed compared with only 10.3% in the placebo group at week 48. All patients in the DTX401 treatment group showed a reduction in cornstarch, with 68% achieving ≥30% reduction and 37% achieving ≥50% reduction.
In stark contrast, only 13% and 4% of the patients in the placebo group achieved ≥30% and ≥50% reductions in cornstarch, respectively, at week 48.
The results from the phase III study are consistent with that of Ultragenyx’s phase I/II study of DTX401 for the same indication.
Year to date, shares of Ultragenyx have plunged 19.2% compared with the industry’s 7.7% decline.
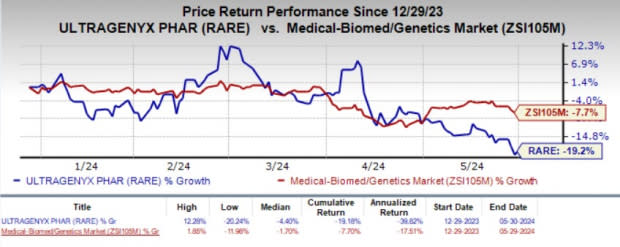
Image Source: Zacks Investment Research
The phase III GlucoGene study also met its key secondary endpoints of reduction in the number of cornstarch doses per day and maintenance of glucose control at week 48. In patients treated with DTX401, a mean reduction of 1.1 cornstarch doses per day was observed compared with a mean reduction of 0.2 in the placebo group. Furthermore, a significant improvement in both the frequency and quantity of nighttime cornstarch dosing was observed in the DTX401 treatment group compared with the placebo group.
A median score of 2.0 (moderately improved) was observed on the Patient Global Impression of Change (PGIC) scale for the DTX401 treatment group compared with a median score of 1.0 (minimally improved) for the placebo group at week 48.
Please note that the PGIC is a self-report measure that reflects a patient’s belief about the efficacy of a treatment. Moderately or higher improved PGIC scores correlated with a ≥30% reduction in total daily cornstarch intake indicated that this is a clinically meaningful threshold for patients.
Additionally, DTX401 demonstrated an acceptable and expected safety profile that is consistent with the phase I/II study results of the candidate for the GSDIa indication.
Ultragenyx expects to present detailed 48-week data from the phase III GlucoGene study at a medical conference later in 2024. The company further plans to discuss these encouraging results with regulatory authorities to support a marketing application in 2025.
Ultragenyx currently has several other gene therapy candidates in its pipeline, which are being evaluated for different indications in separate ongoing clinical-stage studies.
Ultragenyx Pharmaceutical Inc. Price and Consensus
Ultragenyx Pharmaceutical Inc. price-consensus-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank and Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ALX Oncology Holdings ALXO, Annovis Bio ANVS and Entera Bio Ltd. ENTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73. Year to date, shares of ALXO have lost 28.5%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.35 to $2.46. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.82 to $1.95. Year to date, shares of ANVS have plunged 67.9%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Entera Bio’s 2024 loss per share has remained constant at 25 cents. During the same period, the consensus estimate for 2025 loss per share has remained constant at 54 cents. Year to date, shares of ENTX have skyrocketed 285%.
ENTX’s earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 6.50%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
Entera Bio Ltd. (ENTX) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 