Novo Nordisk (NVO) Up 3% as Haemophilia Drug Meets Study Goals
Novo Nordisk NVO achieved co-primary endpoints in a late-stage study evaluating the efficacy and safety of once-weekly and once-monthly subcutaneous Mim8 compared with no prophylaxis and prior coagulation factor prophylaxis treatment in haemophilia A patients aged 12 years or older with or without inhibitors.
Mim8 is NVO’s next-generation FVIIIa mimetic bispecific antibody.
Per the data readout, the phase IIIa FRONTIER 2 study demonstrated a statistically significant and superior reduction of treated bleeding episodes with both once-weekly and once-monthly Mim8 compared with no prophylaxis and prior coagulation factor prophylaxis treatments.
Per management, the results from the study showed the candidate’s potential to prevent bleeding episodes effectively and safely in haemophilia A patients, regardless of their dosing frequency.
The company plans to share detailed data from the phase III FRONTIER program, including the FRONTIER 2 study, at upcoming medical conferences in 2024 and 2025.
Shares of Novo Nordisk gained 3.1% in the last trading session on May 14 as the investors cheered the encouraging results. Year to date, the stock has jumped 28% compared with the industry’s 13.4% growth.
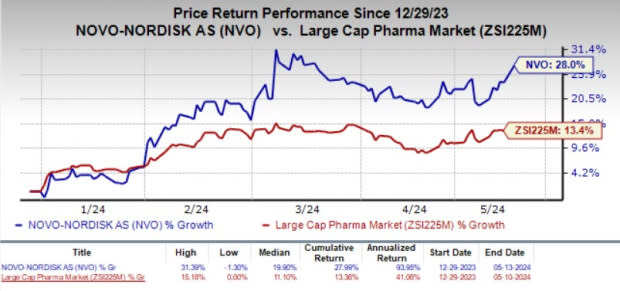
Image Source: Zacks Investment Research
Superior reductions of 97% and 99% in treated bleeds were observed upon treatment with once-weekly and once-monthly Mim8, respectively, in haemophilia A patients with no prior prophylaxis treatment compared with those who received no prophylaxis treatment.
Furthermore, zero treated bleeds were reported in 86% of the patients receiving once-weekly Mim8 and 95% of those receiving once-monthly dosing of the candidate compared with 0% of patients treated with no prophylaxis.
Novo Nordisk also conducted an intra-patient analysis in people with prior coagulation factor prophylaxis. The analysis showed superior reductions of 48% and 43% in treated bleeds upon treatment with the once-weekly and once-monthly Mim8, respectively, compared with prior coagulation factor prophylaxis. Additionally, zero treated bleeds were reported in 66% of people receiving once-weekly Mim8 and 65% of those receiving once-monthly Mim8.
Mim8 was overall well tolerated in the FRONTIER 2 study and demonstrated a safety profile consistent with that observed in previous studies. Treatment with the two dosing regimens of the candidate in the late-stage study did not result in deaths or thromboembolic events (blood clots in the veins).
Novo Nordisk is currently preparing to discuss the phase IIIa FRONTIER 2 study results with regulatory authorities. Based on the feedback from such regulatory bodies, the company plans to submit Mim8 for the first regulatory approval toward the end of 2024.
Haemophilia is a rare genetic disorder that affects the body’s ability to form blood clots,a process needed to stop bleeding. Per NVO, an estimated number of 1,125,000 people worldwide are affected by the disease, out of which approximately 80-85% account for haemophilia A cases.
Novo Nordisk A/S Price and Consensus
Novo Nordisk A/S price-consensus-chart | Novo Nordisk A/S Quote
Zacks Rank & Stocks to Consider
Novo Nordisk currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are Ligand Pharmaceuticals LGND, ANI Pharmaceuticals ANIP and Annovis Bio ANVS, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Ligand’s 2024 earnings per share has remained constant at $4.56. During the same time frame, the consensus estimate for 2025 earnings per share has remained constant at $5.27. Year to date, shares of LGND have gained 17.7%.
Ligand beat estimates in each of the trailing four quarters, delivering an average surprise of 56.02%.
In the past 30 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have risen from $4.43 to $4.44. Meanwhile, during the same period, the consensus estimate for 2025 earnings per share has remained constant at $5.04. Year to date, shares of ANIP have climbed 22.1%.
ANI Pharmaceuticals beat estimates in each of the last four quarters, delivering an average surprise of 53.90%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.35 to $2.93. During the same period, the consensus estimate for 2025 loss per share has widened from $2.82 to $2.83. Year to date, shares of ANVS have plunged 63.4%.
ANVS beat estimates in three of the trailing four quarters and missed the mark on the remaining occasion, delivering an average negative surprise of 1.39%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 