Novavax (NVAX) Seeks Nod for Updated COVID-19 Jab in Europe
Novavax NVAX filed a regulatory application to the EMA, which seeks to update its protein-based COVID-19 vaccine for the 2024/2025 vaccination campaign.
While this updated vaccine has been formulated to target the JN.1 variant, NVAX claims that the vaccine has also been shown to be effective against the current circulating strains, including KP.2 and KP.3.
This submission is in line with the EMA’s guidance issued on Apr 30, which advised COVID-19 vaccine manufacturers like Moderna MRNA, Novavax, and Pfizer PFE to update their respective COVID-19 vaccines to target the JN.1 variant.
Management is working with other regulatory authorities across the globe to secure authorization/approval for updating its protein-based COVID-19 vaccine.
Year to date, the stock has skyrocketed 192.1% against the industry’s 4.1% fall.
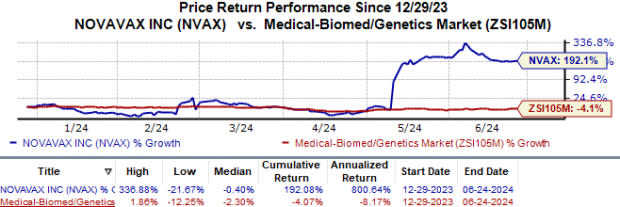
Image Source: Zacks Investment Research
Novavax’s COVID-19 vaccine is only authorized in the European Union (EU) for use in individuals aged 12 years and older. A similar filing was also submitted to the FDA last week, seeking to update its COVID-19 vaccine for the upcoming fall season.
Unlike the EMA guidance, which is in line with the recommendation issued by a World Health Organization (WHO) advisory committee on Apr 26, the FDA updated its guidance advising COVID-19 vaccine makers to target the KP.2 strain, if it is feasible.
The FDA pointed out that post the WHO recommendation, another subvariant named KP.2 has become the dominant strain in the country since the end of April. The latest CDC data (as of Jun 22) shows that the KP.2 and KP.3 strains were the most prevalent, accounting for more than half of COVID-19 cases in the country.
Earlier this month, Moderna also submitted an application to the FDA seeking approval to update its mRNA-based COVID-19 vaccine, for the 2024-2025 fall season. MRNA is simultaneously submitting similar regulatory applications worldwide to support the registration and supply of its vaccine in time for the upcoming vaccination season. Pfizer is yet to provide an update on the FDA filing for its COVID-19 vaccine.
Compared with Pfizer and Moderna, Novavax was not able to reap the benefits of the pandemic due to a delayed launch of its COVID-19 vaccine. During last year’s vaccination season, sales of Novavax suffered due to delayed approval of its vaccine formulation and product launch. We believe that the company’s early participation in the upcoming vaccination season could help it capitalize on the opportunity.
Starting next year, Sanofi SNY will gain rights to co-market Novavax’s protein-based COVID-19 vaccine globally. The French drugmaker will also have the sole license to develop and market the Novavax vaccine in combination with its influenza vaccine. In return, NVAX will be eligible to receive up to $1.2 billion, including $500 million in upfront payment and the rest in milestone payments. It will also be eligible to receive tiered double-digit percentage royalty payments on product sales under this deal. Sanofi also agreed to make an equity investment of nearly $70 million in Novavax in exchange for a 4.9% stake.
The deal with Sanofi breathes new life into Novavax, which had been facing uncertainties in its business for a long time. With the backing of a pharma giant, Novavax expects to increase the market share and presence of its COVID-19 vaccine to a larger audience. This deal even allowed management to do away with its previous concerns about its ability to continue as a ‘going concern.’
Using the funds from the Sanofi deal, Novavax also started making plans to expand its pipeline. Alongside its first-quarter earnings, management claimed to have been developing a new approach for vaccinating against H5N1 bird flu. It is also advancing core technology for different uses like mucosal vaccination and high-density nanoparticles.
Novavax, Inc. Price
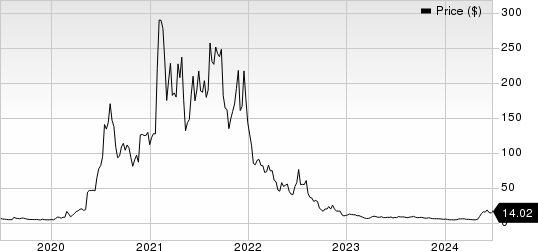
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report

 雅虎香港財經
雅虎香港財經 